Mechanisms of UDCA Non-Response
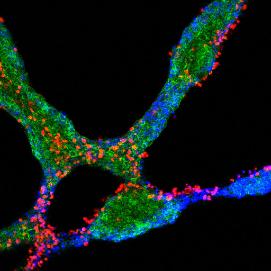
PBC is an autoimmune disease that primarily damages the biliary epithelial cell lining of bile ducts within the liver. It was initially believed this damage rapidly leads to the death of these delicate cells. However, this group has developed an important new model in which immune cells and their target biliary epithelial cells enter a “conversation” leading to a carefully negotiated and gradual outcome which might not involve cell death. Figure 1 shows experimental support for this model with red immune cells and blue-green biliary epithelial cells engaged in dialogue within a tissue culture model of PBC. In essence, we believe that although biliary epithelial cells are stressed during this conversation, many survive. However, the surviving cells are changed in a number of important ways.
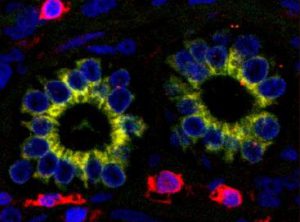
One consequence of the stress in PBC is premature biliary epithelial cell ageing, or senescence. Not only do senescent biliary epithelial cells function less well than their normal counterparts, they produce a range of chemical mediators which communicate their distress to normal cells in the neighbourhood. In their turn, these healthy cells can also lose function by a process called epithelial to mesenchymal transition. As this term implies, the epithelial cells which are crucial for bile duct function can transform into living but functionless mesenchymal cells. The manifest result of this is loss of bile ducts with simultaneous accumulation of the mesenchymal cells characteristic of liver fibrosis. Figure 2 shows biliary epithelial cells within bile ducts expressing a characteristic (yellow) mesenchymal cell marker, demonstrating the initiation of epithelial to mesenchymal transition in a PBC liver biopsy section.
In addition to their impact on neighbouring bile duct cells, stressed epithelial cells can also talk back to the immune cells which infiltrate the liver during PBC. It is becoming ever clearer to immunologists that the functions of these cells are not fixed. Indeed, many can sense their environment and tailor their functions as necessary to maintain good health. Unfortunately this process appears to go awry during autoimmune diseases, tipping the balance away from disease resolution towards continued, chronic tissue damage. In the case of PBC, we believe messages from stressed biliary epithelial cells are responsible not only for mediating the loss of functioning bile ducts but also polarising the local immune response towards aggression, leading to a sustained disease process and further tissue damage.
Workstrand 2 of UK-PBC is focused on the immunobiology of biliary epithelial cells as it is now clear that maintenance or restoration of the health of these vulnerable cells is essential in order to limit the progression of PBC. Fortunately, there are several candidate drugs which have the potential to support the function of biliary epithelial cells. We have already generated pilot data suggesting that the bile acid ursodeoxycholic acid (UDCA or “urso”) can limit production of the chemical drivers of premature cell senescence, thereby breaking the cycle of increasing epithelial cell damage and immune system aggression. We are already planning to investigate how patients with this disease vary in their response to this drug, and whether urso can be supplemented with additional agents to improve its beneficial effects. This study will be combined with a new and very powerful method to examine the mixture of bile acids within specific regions of the liver in order to detect differences between the mixture associated with healthy epithelial cells and those in urso responsive and non-responsive PBC patients.
Further pilot work from our group has shown that biliary epithelial to mesenchymal cell transition has the exciting potential for reversibility if appropriate factors are provided at an early stage. In this study we will determine whether this naturally occurring process of mesenchymal to epithelial transition can be boosted to restore normal functions to bile ducts which have been damaged by PBC. We anticipate that this restoration of bile duct health will have the added advantage of normalising the regulation of otherwise aggressive immune cells within the liver.
In summary this workstrand is built around a new model for PBC which integrates the most recent immunobiological research findings and places the health of bile ducts and their component epithelial cells at the centre stage. We believe that new drug treatments which promote normal functioning of these cells in the face of disease-related stresses will limit the aggressive inflammation, bile duct loss and fibrosis which are all key features observed in patients with urso-unresponsive and progressive PBC. This work will provide robust platforms to test all these possibilities in order to speed the development, optimisation and application of new drugs for treatment of patients with otherwise intractable PBC.

